SMART-LAB
Новый дизайн
Мы делаем деньги на бирже
nyse
NYSE - в этом разделе трейдеры пишут о торговле на американском рынке акций и на нью-йорской фондовой бирже в частности. Здесь, трейдеры американского рынка пишут о своих сделках, выкладывают акции для торговли на сегодняшний день, пишут о новостях по американским компаниям, а также дают технический анализ американского рынка. Чтобы ваши записи по американскому рынку попадали в этот раздел, ставьте тег NYSE своим записям.
Настроение топ-менеджеров (инсайдеров) выжидательное.
- 08 сентября 2014, 11:22
- |

Изменения транзакций по покупкам/продажам среди инсайдеров незначительные за прошедшую неделю. То ли они все уже сказали ранее, то ли просто как и большинство крупных игроков затаились. Хоть их деньги никак не поворачивают рынки, но они всегда стараются быть на шаг его впереди.
Итак
Покупки топ-менеджеров за неделю возросли и составили $22.81 млн по сравнению с предыдущей неделей $18.96млн.
С другой стороны продажи уменьшились и составили $725.84 млн (неделей ранее $932.22млн.)
Sell/Buy соотношение: показатель вычесляется путем деления суммы продаж инсайдерами акций своих компаний за неделю, на сумму потраченную ими на покупку акций своих компаний. Таким образом соотношение за последнюю неделю упало до 31.82 (на прошлой неделе показатель был 49.17)
( Читать дальше )
S&P500 - высокая вероятность флета!
- 07 сентября 2014, 14:45
- |
S&P500 - высокая вероятность флета!
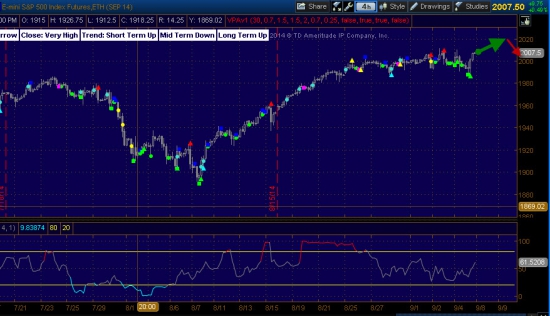
На следующей неделе индекс S&P500 скорее всего будет находится в состоянии флета, в начале недели может подрасти до уровня 2015-2020 с последующим отбитием от этого уровня.

На следующей неделе индекс S&P500 скорее всего будет находится в состоянии флета, в начале недели может подрасти до уровня 2015-2020 с последующим отбитием от этого уровня.
KERX Halt
- 05 сентября 2014, 20:10
- |
KERX захолтан уже час. Когда откроют -будеть возможность рубануть денег на волатильности.
www.nasdaqtrader.com/Trader.aspx?id=Tradehalts
Новость:
NEW YORK, Sept. 5, 2014 (GLOBE NEWSWIRE) — Keryx Biopharmaceuticals, Inc. (Nasdaq: KERX) (the «Company») today announced that the U.S. Food and Drug Administration (FDA) approved Ferric Citrate (formerly known as Zerenex) for the control of serum phosphorus levels in patients with chronic kidney disease (CKD) on dialysis.
«We are thrilled with the FDA's decision to approve Ferric Citrate, and look forward to bringing it to market in the U.S. within the next 12 weeks,» said Ron Bentsur, Chief Executive Officer of Keryx. «We are committed to bringing innovative therapies to the market for patients with kidney disease and are excited to be offering this important treatment option to dialysis patients.»
The U.S. approval of Ferric Citrate was based on data from its Phase 3 registration program. In the Phase 3 clinical trials, Ferric Citrate effectively reduced serum phosphorus levels to well within the KDOQI guidelines range of 3.5 mg/dL to 5.5 mg/dL. In addition to the effects on serum phosphorus levels, Ferric Citrate's pharmacodynamic properties resulted in increased ferritin and transferrin saturation (TSAT); whereas these parameters remained relatively constant in patients treated with active control (Renvela® and/or Phoslo®). The most common adverse events for Ferric Citrate treated patients were gastrointestinal-related, including diarrhea, nausea, vomiting and constipation.
«I believe that Ferric Citrate offers clear benefits to patients and represents a new way for physicians to manage hyperphosphatemia,» said Julia Lewis, MD, lead investigator, nephrologist and Professor of Medicine at Vanderbilt University Medical Center. «Given Ferric Citrate's pharmacodynamic properties that lead to increases in iron stores, physicians should assess and monitor iron parameters and may need to reduce the dose of or discontinue IV iron therapy.»
( Читать дальше )
www.nasdaqtrader.com/Trader.aspx?id=Tradehalts
Новость:
NEW YORK, Sept. 5, 2014 (GLOBE NEWSWIRE) — Keryx Biopharmaceuticals, Inc. (Nasdaq: KERX) (the «Company») today announced that the U.S. Food and Drug Administration (FDA) approved Ferric Citrate (formerly known as Zerenex) for the control of serum phosphorus levels in patients with chronic kidney disease (CKD) on dialysis.
«We are thrilled with the FDA's decision to approve Ferric Citrate, and look forward to bringing it to market in the U.S. within the next 12 weeks,» said Ron Bentsur, Chief Executive Officer of Keryx. «We are committed to bringing innovative therapies to the market for patients with kidney disease and are excited to be offering this important treatment option to dialysis patients.»
The U.S. approval of Ferric Citrate was based on data from its Phase 3 registration program. In the Phase 3 clinical trials, Ferric Citrate effectively reduced serum phosphorus levels to well within the KDOQI guidelines range of 3.5 mg/dL to 5.5 mg/dL. In addition to the effects on serum phosphorus levels, Ferric Citrate's pharmacodynamic properties resulted in increased ferritin and transferrin saturation (TSAT); whereas these parameters remained relatively constant in patients treated with active control (Renvela® and/or Phoslo®). The most common adverse events for Ferric Citrate treated patients were gastrointestinal-related, including diarrhea, nausea, vomiting and constipation.
«I believe that Ferric Citrate offers clear benefits to patients and represents a new way for physicians to manage hyperphosphatemia,» said Julia Lewis, MD, lead investigator, nephrologist and Professor of Medicine at Vanderbilt University Medical Center. «Given Ferric Citrate's pharmacodynamic properties that lead to increases in iron stores, physicians should assess and monitor iron parameters and may need to reduce the dose of or discontinue IV iron therapy.»
( Читать дальше )
SPY упал на премаркете
- 05 сентября 2014, 17:48
- |
Ближайший уровень поддержки по SPY – 199.50 уровень сопротивления – 200.30

COST покупка при ужержании 125
EMES покупка выше 125
EGN смотрим на разворот
Gapping up/down: BLOX +13% and AMBA +9% after earnings, PRAN +28% after orphan designation status, CIEN +2% after upgrade; GPX -6% and FNSR -5% after earnings/SSS, KORS -5% after offering
Gapping up
In reaction to strong earnings/guidance: BLOX +13.4%, AMBA +9.3%, LOCO +2.9%, VRNT +2.6%.
Other news: PRAN +27.6% (received orphan designation status for its product for the treatment of Huntington's disease), HART +13.7% (received orphan designation status for its inBreath airway transplant system to restore the structure and/or function of the trachea subsequent to tracheal damage due to cancer, injury or infection), NSPH +10.8% (has engaged Jefferies LLC as its financial advisor to assist it in the development and evaluation of a full range of potential strategic alternatives for the Company), TASR +6% (higher following announcement that the NYPD will begin a pilot program of equipping officers with body cameras), OMED +4.1% (FDA removes partial clinical hold on OncoMed's ipafricept), ICLD +2.8% (announces multi-site deployment of security and threat prevention solutions and services for a large global consulting firm), MOBI +2.6% (announces repurchase of 16 mln shares from Sequoia Capital ), GPRO +1.7% (up in sympathy following supplier AMBA earnings), DGLY +1.5% (cont usual volatility pre-mkt), JOY +1% (positive Barron's mention), SD +0.8% ( announces $200 mln share repurchase program)
( Читать дальше )

COST покупка при ужержании 125
EMES покупка выше 125
EGN смотрим на разворот
Gapping up/down: BLOX +13% and AMBA +9% after earnings, PRAN +28% after orphan designation status, CIEN +2% after upgrade; GPX -6% and FNSR -5% after earnings/SSS, KORS -5% after offering
Gapping up
In reaction to strong earnings/guidance: BLOX +13.4%, AMBA +9.3%, LOCO +2.9%, VRNT +2.6%.
Other news: PRAN +27.6% (received orphan designation status for its product for the treatment of Huntington's disease), HART +13.7% (received orphan designation status for its inBreath airway transplant system to restore the structure and/or function of the trachea subsequent to tracheal damage due to cancer, injury or infection), NSPH +10.8% (has engaged Jefferies LLC as its financial advisor to assist it in the development and evaluation of a full range of potential strategic alternatives for the Company), TASR +6% (higher following announcement that the NYPD will begin a pilot program of equipping officers with body cameras), OMED +4.1% (FDA removes partial clinical hold on OncoMed's ipafricept), ICLD +2.8% (announces multi-site deployment of security and threat prevention solutions and services for a large global consulting firm), MOBI +2.6% (announces repurchase of 16 mln shares from Sequoia Capital ), GPRO +1.7% (up in sympathy following supplier AMBA earnings), DGLY +1.5% (cont usual volatility pre-mkt), JOY +1% (positive Barron's mention), SD +0.8% ( announces $200 mln share repurchase program)
( Читать дальше )
Запоздалые инвесторы, перестаньте обманывать себя!
- 05 сентября 2014, 16:27
- |

Многие частные инвесторы продолжают ждать удобного момента (точнее сильного отката), что бы войти в лодку быков. Как всегда одна и таже отговорка — «Я выжидаю настоящую коррекцию».
Речь идет не о тех, кто уже давно в нужном тренде. Я говорю о сомневающихся. Постоянное ожидание коррекции процентов на 10% оттягивает их момент все дальше и дальше.
Но сейчас как раз у этих ребят и возникнет шанс купить на очень долгий срок, как они думают. На самом же деле, упав на 10%, экономике США уже может не хватить времени для демонстрации долгосрочного бычьего потенциала.
Как я уже говорил, 2015 не самый лучший год для удержания длинных позиций.
Индекс СиПИ500 не падал на столь большую цифру начиная с июня 2012-го. Если же падение произойдет именно сейчас, на борт мы получим много мяса средненьких инвесторов. Они будут думать, что делают деньги, на самом е деле может так получиться, что делают их.
Apple - время покупать!!!
- 05 сентября 2014, 13:02
- |
Apple Inc — американская корпорация, производитель персональных и планшетных компьютеров, аудиоплееров, телефонов, программного обеспечения. Один из пионеров в области персональных компьютеров и современных многозадачных операционных систем с графическим интерфейсом. Штаб-квартира — в Купертино, штат Калифорния.
Благодаря инновационным технологиям и эстетичному дизайну, корпорация Apple создала уникальную репутацию, сравнимую с культом, в индустрии потребительской электроники. Компания прошла нелегкий путь развития. Ключевой фигурой стал отец-основатель компании Стив Джобс, благодаря которому компания начала существовать. После смерти Стива многие задавались вопросом: «Сможет ли компания продолжить занимать лидирующие позиции на рынке смартфонов?». За последний год акции компании выросли с 480 долларов (цена была перед презентацией iPhone 5s и iPhone 5c) до 714 $ в начале сентября (после сплита 102$).Феноменальный рост объясняется в первую очередь ростом продаж новых айфонов, которые превысили ожидания, и продолжением программы выкупа акций. Негативом было снижение популярности планшетов iPad .
( Читать дальше )
Благодаря инновационным технологиям и эстетичному дизайну, корпорация Apple создала уникальную репутацию, сравнимую с культом, в индустрии потребительской электроники. Компания прошла нелегкий путь развития. Ключевой фигурой стал отец-основатель компании Стив Джобс, благодаря которому компания начала существовать. После смерти Стива многие задавались вопросом: «Сможет ли компания продолжить занимать лидирующие позиции на рынке смартфонов?». За последний год акции компании выросли с 480 долларов (цена была перед презентацией iPhone 5s и iPhone 5c) до 714 $ в начале сентября (после сплита 102$).Феноменальный рост объясняется в первую очередь ростом продаж новых айфонов, которые превысили ожидания, и продолжением программы выкупа акций. Негативом было снижение популярности планшетов iPad .
( Читать дальше )
- bitcoin
- brent
- eurusd
- forex
- gbpusd
- gold
- imoex
- ipo
- nasdaq
- nyse
- rts
- s&p500
- si
- usdrub
- wti
- акции
- алготрейдинг
- алроса
- аналитика
- аэрофлот
- банки
- биржа
- биткоин
- брокеры
- валюта
- вдо
- волновой анализ
- волны эллиотта
- вопрос
- втб
- газ
- газпром
- гмк норникель
- дивиденды
- доллар
- доллар рубль
- евро
- золото
- инвестиции
- индекс мб
- инфляция
- китай
- коронавирус
- кризис
- криптовалюта
- лидеры роста и падения ммвб
- лукойл
- магнит
- ммвб
- мобильный пост
- мосбиржа
- московская биржа
- мтс
- нефть
- новатэк
- новости
- обзор рынка
- облигации
- опрос
- опционы
- отчеты мсфо
- офз
- оффтоп
- прогноз
- прогноз по акциям
- раскрытие информации
- ри
- роснефть
- россия
- ртс
- рубль
- рынки
- рынок
- санкции
- сбер
- сбербанк
- северсталь
- си
- сигналы
- смартлаб
- сущфакты
- сша
- технический анализ
- торговля
- торговые роботы
- торговые сигналы
- трейдер
- трейдинг
- украина
- фондовый рынок
- форекс
- фрс
- фьючерс
- фьючерс mix
- фьючерс ртс
- фьючерсы
- цб
- экономика
- юмор
- яндекс


















 Новости тг-канал
Новости тг-канал