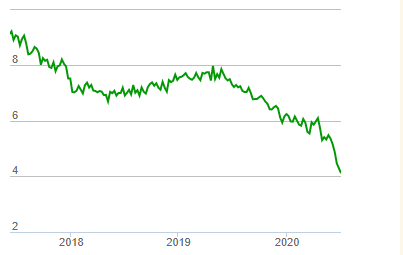
Купил по 11.85 вчера, компания занимается созданием лекарства от рассеянного склероза, 17 июня вышла новость
Based on promising results from the Phase 1a study including sustained disability improvements among progressive MS patients, the Phase 1b study will assess clinical outcomes including measures of disability, physical and cognitive function, biomarkers and MRI imaging as well as safety
Atara Biotherapeutics, Inc. (Nasdaq: ATRA), a pioneer in T-cell immunotherapy leveraging its novel allogeneic EBV T-cell platform to develop treatments for patients with severe diseases including solid tumors, hematologic cancers and autoimmune disease, today announced the first patient has been enrolled in a Phase 1b study of ATA188, an off-the-shelf, allogeneic EBV-specific T-cell immunotherapy, in patients with progressive forms of multiple sclerosis (MS).
The Phase 1b study (NCT03283826) is a double-blind randomized placebo-controlled study evaluating the efficacy and safety of ATA188. In addition to measuring change in disability measures compared to baseline, especially sustained improvement over time, the Phase 1b study will also include multiple measures of patients’ function as well as various biomarkers. Types of data to be collected include: standard disability measures (EDSS, timed 25 foot walk test, 9 hole peg test); cognition and outpatient ambulatory activity; patient-reported fatigue; visual acuity; biomarkers in blood and cerebrospinal fluid (CSF); and, MRI imaging.
«We are thrilled at the investigator and patient interest in our randomized placebo-controlled Phase 1b study assessing the efficacy and safety of ATA188,» said AJ Joshi, MD, Senior Vice President and Chief Medical Officer of Atara Biotherapeutics. «The initiation of this study, including first-patient-enrolled, is an important step in further assessing the potential of ATA188 in progressive MS, a complex disease of high unmet medical need where disability continues to progress despite approved treatment options. Based on the promising results of safety and sustained disability improvements seen to date in the Phase 1a study, we look forward to continuing enrollment in this randomized placebo-controlled study with the goal of developing a transformative therapy for patients that could halt or reverse the progression of this severe disease.»
Participants in clinical sites across the U.S. and Australia will be randomized to receive ATA188 or matching placebo at the cohort 3 dose selected from the Phase 1a portion of the study. The primary end point will be evaluated at 12 months, after which, all subjects will be switched to active treatment and followed for an additional 12 months. All subjects will have the opportunity to enter into a three-year open-label extension after the first two years of treatment.
Progression into the randomized, placebo-controlled study of ATA188 is supported by data from the Phase 1a study that were featured in a late-breaking e-poster at the 6th Congress of the European Academy of Neurology (EAN) virtual meeting, held May 23-26, 2020. These data demonstrate that ATA188 was safe and well-tolerated across all four dose cohorts. Importantly, there was a higher proportion of patients showing sustained disability improvements with increasing dose, and sustained disability improvements seen at six months were maintained at 12 months in all three cohorts that have reached the 12-month time point in the study.
In addition to the clinical data reported at EAN, preclinical translational data recently published online at the 2020 Academy of Neurology (AAN) 72nd Annual Meeting further support the proposed mechanism of action of ATA188. These analyses of the T-cells comprising ATA188 demonstrate that they recognize MS-relevant EBV antigens via defined T-cell receptors. This supports the proposed mechanism of action of ATA188 in targeting the MS-relevant antigens on EBV-infected B cells believed to play an important role in propagating the autoimmune cascade that causes MS.
About Multiple Sclerosis (MS)
MS is a chronic neurological autoimmune disease that affects an estimated 2.3 million people around the world. Relapsing-remitting MS (RRMS) is the most common form of MS and is characterized by episodes of new or worsening signs or symptoms (relapses) followed by periods of recovery. Despite available disease-modifying treatments, most individuals with RRMS continue to experience disease activity and disability progression.